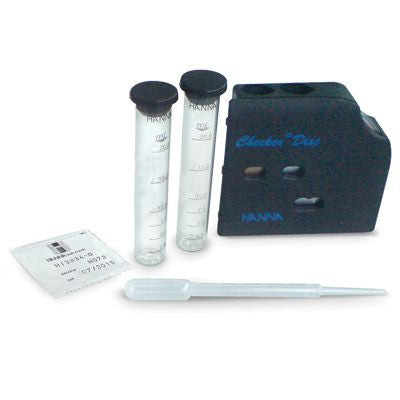
{"id":1582107333,"title":"Hanna CTK Manganese","handle":"hi38042","description":"\u003cp\u003eManganese is not present in natural waters but it is found in various salts and minerals frequently associated with iron compounds. Manganese salts are used as fertilizer additives. in ferroalloys (in steel manufacture). in nonferrous alloys as it improves their corrosion resistance and hardness.\u003c\/p\u003e\n\u003cp\u003eThe HANNA test kit determines manganese concentration via a checker disc. The reaction between manganese and reagents causes a violet tint in the sample which is proportional to the manganese concentration.\u003c\/p\u003e\n\u003cp\u003e \u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003e HI 38042 test kit comes with 100 packets of buffer reagent A. 100 packets manganese reagent B. checker disc. glass vials with caps (2) and 3 mL plastic pipette.\u003c\/p\u003e\n\u003cdiv\u003e\u003cstrong\u003eProduct Manuals\u003c\/strong\u003e\u003c\/div\u003e\n\u003cp\u003e\u003cimg width=\"20\" height=\"22\" align=\"absmiddle\" alt=\"\" src=\"http:\/\/www.hannainst.com\/usa\/images\/icon-pdf.gif\" border=\"0\"\u003e Manual: \u003ca title=\"Download Product Manual\" href=\"http:\/\/www.hannainst.com\/manuals\/manHI_38042.pdf\" target=\"_blank\" rel=\"noopener noreferrer\"\u003eDownload\u003c\/a\u003e\u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eSpecification :\u003c\/strong\u003e\u003c\/p\u003e\n\u003ctable class=\"spec_table mceItemTable\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eChecker Disc\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.0-3.0 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.2 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eSodium Periodate\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e100\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e560 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cp\u003e \u003c\/p\u003e","published_at":"2016-09-01T16:03:00+12:00","created_at":"2015-09-01T15:08:36+12:00","vendor":"Hanna Instruments","type":"Electrochemical","tags":["Category_Other","Manufacturer_Hanna Instruments"],"price":24000,"price_min":24000,"price_max":24000,"available":true,"price_varies":false,"compare_at_price":null,"compare_at_price_min":0,"compare_at_price_max":0,"compare_at_price_varies":false,"variants":[{"id":4857180869,"title":"Default Title","option1":"Default Title","option2":null,"option3":null,"sku":"HI38042","requires_shipping":false,"taxable":true,"featured_image":null,"available":true,"name":"Hanna CTK Manganese","public_title":null,"options":["Default Title"],"price":24000,"weight":0,"compare_at_price":null,"inventory_quantity":1,"inventory_management":null,"inventory_policy":"deny","barcode":null,"requires_selling_plan":false,"selling_plan_allocations":[]}],"images":["\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0000ea8808_2015_f286d16e-875e-47a8-94e2-4a7508d9e769.jpeg?v=1467968223"],"featured_image":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0000ea8808_2015_f286d16e-875e-47a8-94e2-4a7508d9e769.jpeg?v=1467968223","options":["Title"],"media":[{"alt":"CTK Manganese - Acorn Scientific","id":21279768675,"position":1,"preview_image":{"aspect_ratio":1.0,"height":400,"width":400,"src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0000ea8808_2015_f286d16e-875e-47a8-94e2-4a7508d9e769.jpeg?v=1467968223"},"aspect_ratio":1.0,"height":400,"media_type":"image","src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0000ea8808_2015_f286d16e-875e-47a8-94e2-4a7508d9e769.jpeg?v=1467968223","width":400}],"requires_selling_plan":false,"selling_plan_groups":[],"content":"\u003cp\u003eManganese is not present in natural waters but it is found in various salts and minerals frequently associated with iron compounds. Manganese salts are used as fertilizer additives. in ferroalloys (in steel manufacture). in nonferrous alloys as it improves their corrosion resistance and hardness.\u003c\/p\u003e\n\u003cp\u003eThe HANNA test kit determines manganese concentration via a checker disc. The reaction between manganese and reagents causes a violet tint in the sample which is proportional to the manganese concentration.\u003c\/p\u003e\n\u003cp\u003e \u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003e HI 38042 test kit comes with 100 packets of buffer reagent A. 100 packets manganese reagent B. checker disc. glass vials with caps (2) and 3 mL plastic pipette.\u003c\/p\u003e\n\u003cdiv\u003e\u003cstrong\u003eProduct Manuals\u003c\/strong\u003e\u003c\/div\u003e\n\u003cp\u003e\u003cimg width=\"20\" height=\"22\" align=\"absmiddle\" alt=\"\" src=\"http:\/\/www.hannainst.com\/usa\/images\/icon-pdf.gif\" border=\"0\"\u003e Manual: \u003ca title=\"Download Product Manual\" href=\"http:\/\/www.hannainst.com\/manuals\/manHI_38042.pdf\" target=\"_blank\" rel=\"noopener noreferrer\"\u003eDownload\u003c\/a\u003e\u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eSpecification :\u003c\/strong\u003e\u003c\/p\u003e\n\u003ctable class=\"spec_table mceItemTable\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eChecker Disc\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.0-3.0 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.2 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eSodium Periodate\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e100\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e560 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cp\u003e \u003c\/p\u003e"}