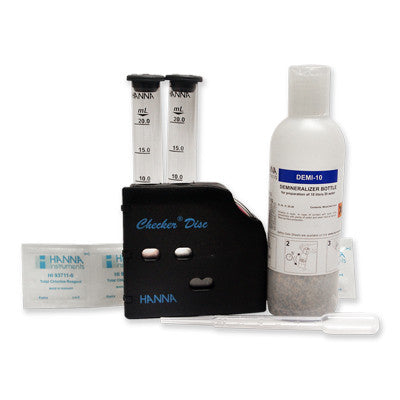
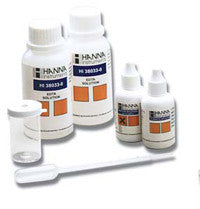
{"id":2012214597,"title":"HANNA HI 3843 Hypochlorite Test Kit","handle":"hi-3843","description":"\u003cp\u003e \u003c\/p\u003e\n\u003cdiv id=\"Product-Body\" style=\"width: 475px; clear: left; color: #222222; font-family: Tahoma. Arial. FreeSans. Helvetica. sans-serif; font-size: 12px; line-height: 18px; background-color: #f2f3f3;\"\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eThe available chlorine refers to the chlorine liberated by the action of dilute acid on the hypochlorite:\u003cbr\u003e(OCl)\u003csup\u003e—\u003c\/sup\u003e + Cl\u003csup\u003e—\u003c\/sup\u003e + 2H\u003csup\u003e+\u003c\/sup\u003e → Cl\u003csub\u003e2\u003c\/sub\u003e + H\u003csub\u003e2\u003c\/sub\u003eO\u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eAn iodometric titration method is used in this test kit. The hypochlorite solution is treated with potassium iodide and strongly acidified with acid:\u003cbr\u003e(OCl)\u003csup\u003e—\u003c\/sup\u003e + 2H\u003csup\u003e+\u003c\/sup\u003e + 2I\u003csup\u003e—\u003c\/sup\u003e → Cl\u003csup\u003e—\u003c\/sup\u003e + I\u003csub\u003e2\u003c\/sub\u003e + H\u003csub\u003e2\u003c\/sub\u003eO\u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eThe amount of iodine generated is equivalent to the chlorine in the sample. The concentration of iodine is then calculated by titration of thiosulfate ions that reduce the iodine back to iodide ions: \u003cbr\u003eI\u003csub\u003e2\u003c\/sub\u003e + 2(S\u003csub\u003e2\u003c\/sub\u003eO\u003csub\u003e3\u003c\/sub\u003e)\u003csup\u003e2–\u003c\/sup\u003e → 2I\u003csup\u003e—\u003c\/sup\u003e + (S\u003csub\u003e4\u003c\/sub\u003eO\u003csub\u003e6\u003c\/sub\u003e)\u003csup\u003e2-\u003c\/sup\u003e\u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003e \u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003eHI 3843 test kit comes with 30 mL potassium iodide solution. 100 packets bleach reagent B. 60 mL bleach reagent C (2). 125 mL glass Erlenmeyer flask and 1 mL plastic pipettes (25).\u003c\/p\u003e\n\u003c\/div\u003e\n\u003cdiv id=\"TabbedPanels1\" class=\"TabbedPanels\" style=\"margin: 0px; padding: 0px; float: left; clear: none; width: 456.9375px; color: #222222; font-family: Tahoma. Arial. FreeSans. Helvetica. sans-serif; font-size: 12px; line-height: 18px; background-color: #f2f3f3;\"\u003e\n\u003cul class=\"TabbedPanelsTabGroup\" style=\"margin: 0px 0px 0px 20px; padding: 0px;\"\u003e\n\u003cli class=\"TabbedPanelsTab TabbedPanelsTabSelected\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #eeeeee; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #eeeeee #cccccc; cursor: pointer; font-size: 11px;\"\u003eSpecifications\u003c\/li\u003e\n\u003cli class=\"TabbedPanelsTab\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #dddddd; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #999999 #cccccc; cursor: pointer; font-size: 11px;\"\u003eMSDS\u003c\/li\u003e\n\u003cli class=\"TabbedPanelsTab\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #dddddd; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #999999 #cccccc; cursor: pointer; font-size: 11px;\"\u003eAccessories\u003c\/li\u003e\n\u003cli class=\"TabbedPanelsTab\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #dddddd; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #999999 #cccccc; cursor: pointer; font-size: 11px;\"\u003eDownloads\u003c\/li\u003e\n\u003c\/ul\u003e\n\u003cdiv class=\"TabbedPanelsContentGroup\" style=\"clear: both; border-style: solid; border-width: 1px; border-color: #999999 #999999 #cccccc #cccccc; background-color: #eeeeee;\"\u003e\n\u003cdiv class=\"TabbedPanelsContent TabbedPanelsContentVisible\" style=\"padding: 4px;\"\u003e\n\u003ctable class=\"spec_table\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eTitration\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e50-150 g\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e5 g\/L (0.5%)\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eIodometric\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eapprox. 100\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e485 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eClick on the links below to download the Safety Data Sheet.\u003c\/p\u003e\n\u003cul style=\"margin: 0px 0px 0px 20px;\"\u003e\n\u003cli\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/sds\/SDS_HI%203843B-0_2012-05-21.pdf\"\u003eHI 3843B-0\u003c\/a\u003e\u003c\/li\u003e\n\u003cli\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/sds\/SDS_HI%203843C-0_2013-07-01.pdf\"\u003eHI 3843C-0\u003c\/a\u003e\u003c\/li\u003e\n\u003cli\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/sds\/SDS_POTASSIODIDE_2013-06-27.pdf\"\u003ePOTASSIODIDE\u003c\/a\u003e\u003c\/li\u003e\n\u003c\/ul\u003e\n\u003ctable class=\"spec_table\" style=\"color: #222222; font-size: 12px; line-height: 18px;\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\u003cth\u003eReagents \u0026amp; Standards\u003c\/th\u003e\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/usa\/prods2.cfm?id=001001\u0026amp;ProdCode=HI%203843-100\"\u003eHI 3843-100\u003c\/a\u003e\u003c\/td\u003e\n\u003ctd\u003eHypochlorite (Cl\u003csub\u003e2\u003c\/sub\u003e)\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cdiv style=\"background-color: #f2f3f3;\"\u003e\u003cstrong\u003eProduct Manuals\u003c\/strong\u003e\u003c\/div\u003e\n\u003cp\u003e\u003cimg style=\"background-color: #f2f3f3;\" src=\"http:\/\/www.hannainst.com\/usa\/images\/icon-pdf.gif\" alt=\"\" width=\"20\" height=\"22\" align=\"absmiddle\" border=\"0\"\u003e\u003cspan style=\"background-color: #f2f3f3;\"\u003e Manual: \u003c\/span\u003e\u003ca style=\"color: #0066cc; font-weight: bold; background-color: #f2f3f3;\" title=\"Download Product Manual\" href=\"http:\/\/www.hannainst.com\/manuals\/manHI_3843.pdf\" target=\"_blank\"\u003eDownload\u003c\/a\u003e\u003c\/p\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e","published_at":"2016-09-01T16:07:58+12:00","created_at":"2015-09-04T15:27:41+12:00","vendor":"Hanna Instruments","type":"Electrochemical","tags":["Category_pH","Manufacturer_Hanna Instruments","Type_Buffers"],"price":18000,"price_min":18000,"price_max":18000,"available":true,"price_varies":false,"compare_at_price":null,"compare_at_price_min":0,"compare_at_price_max":0,"compare_at_price_varies":false,"variants":[{"id":5764603909,"title":"Default Title","option1":"Default Title","option2":null,"option3":null,"sku":"HI 3843","requires_shipping":false,"taxable":true,"featured_image":null,"available":true,"name":"HANNA HI 3843 Hypochlorite Test Kit","public_title":null,"options":["Default Title"],"price":18000,"weight":0,"compare_at_price":null,"inventory_quantity":1,"inventory_management":null,"inventory_policy":"deny","barcode":null,"requires_selling_plan":false,"selling_plan_allocations":[]}],"images":["\/\/acornsci.co.nz\/cdn\/shop\/products\/download_e75c83f9-9982-4a6f-9405-dd3f47751fdf.jpg?v=1573506133"],"featured_image":"\/\/acornsci.co.nz\/cdn\/shop\/products\/download_e75c83f9-9982-4a6f-9405-dd3f47751fdf.jpg?v=1573506133","options":["Title"],"media":[{"alt":"HANNA HI 3843 Hypochlorite Test Kit - Acorn Scientific","id":5732153983075,"position":1,"preview_image":{"aspect_ratio":1.0,"height":225,"width":225,"src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/download_e75c83f9-9982-4a6f-9405-dd3f47751fdf.jpg?v=1573506133"},"aspect_ratio":1.0,"height":225,"media_type":"image","src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/download_e75c83f9-9982-4a6f-9405-dd3f47751fdf.jpg?v=1573506133","width":225}],"requires_selling_plan":false,"selling_plan_groups":[],"content":"\u003cp\u003e \u003c\/p\u003e\n\u003cdiv id=\"Product-Body\" style=\"width: 475px; clear: left; color: #222222; font-family: Tahoma. Arial. FreeSans. Helvetica. sans-serif; font-size: 12px; line-height: 18px; background-color: #f2f3f3;\"\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eThe available chlorine refers to the chlorine liberated by the action of dilute acid on the hypochlorite:\u003cbr\u003e(OCl)\u003csup\u003e—\u003c\/sup\u003e + Cl\u003csup\u003e—\u003c\/sup\u003e + 2H\u003csup\u003e+\u003c\/sup\u003e → Cl\u003csub\u003e2\u003c\/sub\u003e + H\u003csub\u003e2\u003c\/sub\u003eO\u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eAn iodometric titration method is used in this test kit. The hypochlorite solution is treated with potassium iodide and strongly acidified with acid:\u003cbr\u003e(OCl)\u003csup\u003e—\u003c\/sup\u003e + 2H\u003csup\u003e+\u003c\/sup\u003e + 2I\u003csup\u003e—\u003c\/sup\u003e → Cl\u003csup\u003e—\u003c\/sup\u003e + I\u003csub\u003e2\u003c\/sub\u003e + H\u003csub\u003e2\u003c\/sub\u003eO\u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eThe amount of iodine generated is equivalent to the chlorine in the sample. The concentration of iodine is then calculated by titration of thiosulfate ions that reduce the iodine back to iodide ions: \u003cbr\u003eI\u003csub\u003e2\u003c\/sub\u003e + 2(S\u003csub\u003e2\u003c\/sub\u003eO\u003csub\u003e3\u003c\/sub\u003e)\u003csup\u003e2–\u003c\/sup\u003e → 2I\u003csup\u003e—\u003c\/sup\u003e + (S\u003csub\u003e4\u003c\/sub\u003eO\u003csub\u003e6\u003c\/sub\u003e)\u003csup\u003e2-\u003c\/sup\u003e\u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003e \u003c\/p\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003eHI 3843 test kit comes with 30 mL potassium iodide solution. 100 packets bleach reagent B. 60 mL bleach reagent C (2). 125 mL glass Erlenmeyer flask and 1 mL plastic pipettes (25).\u003c\/p\u003e\n\u003c\/div\u003e\n\u003cdiv id=\"TabbedPanels1\" class=\"TabbedPanels\" style=\"margin: 0px; padding: 0px; float: left; clear: none; width: 456.9375px; color: #222222; font-family: Tahoma. Arial. FreeSans. Helvetica. sans-serif; font-size: 12px; line-height: 18px; background-color: #f2f3f3;\"\u003e\n\u003cul class=\"TabbedPanelsTabGroup\" style=\"margin: 0px 0px 0px 20px; padding: 0px;\"\u003e\n\u003cli class=\"TabbedPanelsTab TabbedPanelsTabSelected\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #eeeeee; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #eeeeee #cccccc; cursor: pointer; font-size: 11px;\"\u003eSpecifications\u003c\/li\u003e\n\u003cli class=\"TabbedPanelsTab\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #dddddd; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #999999 #cccccc; cursor: pointer; font-size: 11px;\"\u003eMSDS\u003c\/li\u003e\n\u003cli class=\"TabbedPanelsTab\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #dddddd; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #999999 #cccccc; cursor: pointer; font-size: 11px;\"\u003eAccessories\u003c\/li\u003e\n\u003cli class=\"TabbedPanelsTab\" style=\"position: relative; top: 1px; float: left; padding: 2px 4px; margin: 0px 1px 0px 0px; font-weight: bold; line-height: normal; font-family: sans-serif; background-color: #dddddd; list-style: none; border-style: solid; border-width: 1px; border-color: #999999 #999999 #999999 #cccccc; cursor: pointer; font-size: 11px;\"\u003eDownloads\u003c\/li\u003e\n\u003c\/ul\u003e\n\u003cdiv class=\"TabbedPanelsContentGroup\" style=\"clear: both; border-style: solid; border-width: 1px; border-color: #999999 #999999 #cccccc #cccccc; background-color: #eeeeee;\"\u003e\n\u003cdiv class=\"TabbedPanelsContent TabbedPanelsContentVisible\" style=\"padding: 4px;\"\u003e\n\u003ctable class=\"spec_table\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eTitration\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e50-150 g\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e5 g\/L (0.5%)\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eIodometric\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eapprox. 100\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e485 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cp style=\"margin: 0px 0px 15px;\"\u003eClick on the links below to download the Safety Data Sheet.\u003c\/p\u003e\n\u003cul style=\"margin: 0px 0px 0px 20px;\"\u003e\n\u003cli\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/sds\/SDS_HI%203843B-0_2012-05-21.pdf\"\u003eHI 3843B-0\u003c\/a\u003e\u003c\/li\u003e\n\u003cli\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/sds\/SDS_HI%203843C-0_2013-07-01.pdf\"\u003eHI 3843C-0\u003c\/a\u003e\u003c\/li\u003e\n\u003cli\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/sds\/SDS_POTASSIODIDE_2013-06-27.pdf\"\u003ePOTASSIODIDE\u003c\/a\u003e\u003c\/li\u003e\n\u003c\/ul\u003e\n\u003ctable class=\"spec_table\" style=\"color: #222222; font-size: 12px; line-height: 18px;\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\u003cth\u003eReagents \u0026amp; Standards\u003c\/th\u003e\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003e\u003ca style=\"color: #3300ff; text-decoration: none; font-weight: bold;\" href=\"http:\/\/www.hannainst.com\/usa\/prods2.cfm?id=001001\u0026amp;ProdCode=HI%203843-100\"\u003eHI 3843-100\u003c\/a\u003e\u003c\/td\u003e\n\u003ctd\u003eHypochlorite (Cl\u003csub\u003e2\u003c\/sub\u003e)\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cdiv style=\"background-color: #f2f3f3;\"\u003e\u003cstrong\u003eProduct Manuals\u003c\/strong\u003e\u003c\/div\u003e\n\u003cp\u003e\u003cimg style=\"background-color: #f2f3f3;\" src=\"http:\/\/www.hannainst.com\/usa\/images\/icon-pdf.gif\" alt=\"\" width=\"20\" height=\"22\" align=\"absmiddle\" border=\"0\"\u003e\u003cspan style=\"background-color: #f2f3f3;\"\u003e Manual: \u003c\/span\u003e\u003ca style=\"color: #0066cc; font-weight: bold; background-color: #f2f3f3;\" title=\"Download Product Manual\" href=\"http:\/\/www.hannainst.com\/manuals\/manHI_3843.pdf\" target=\"_blank\"\u003eDownload\u003c\/a\u003e\u003c\/p\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e"}