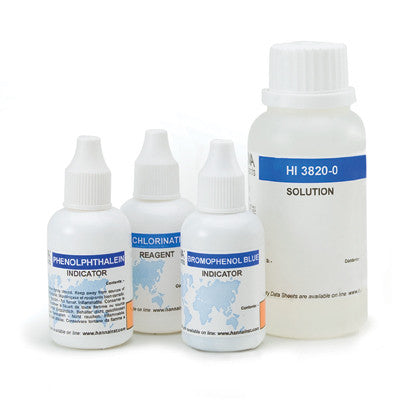
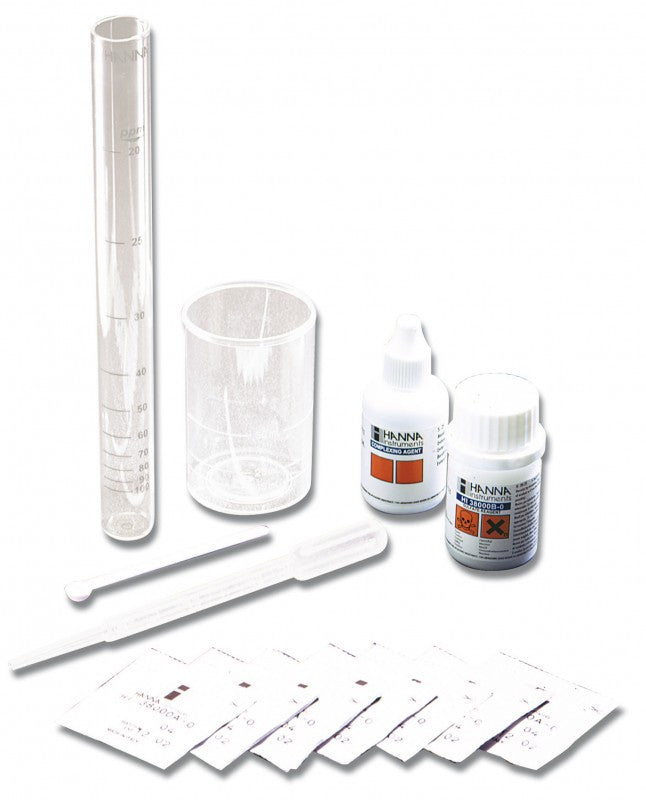
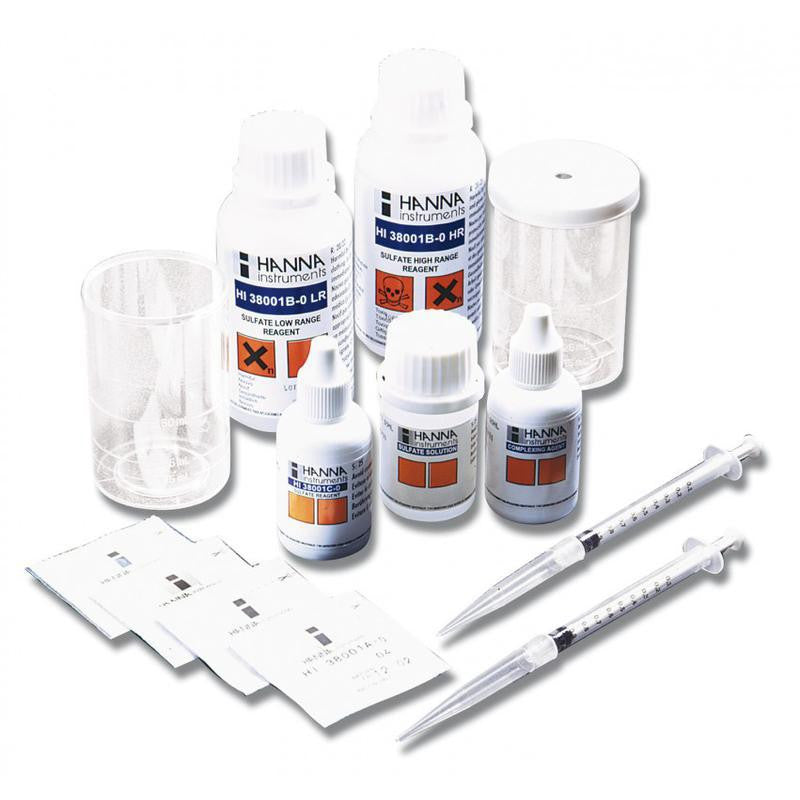
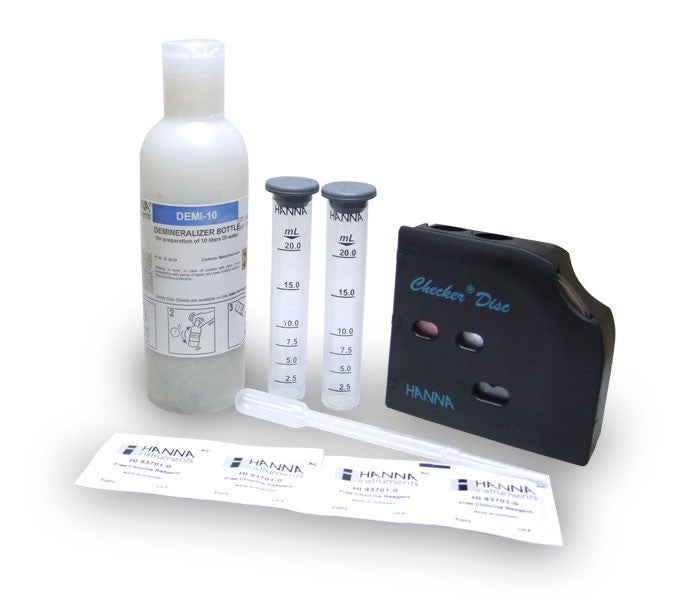
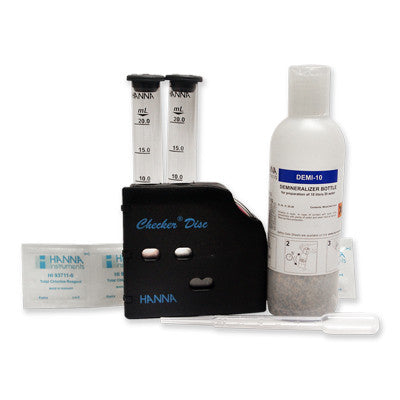
{"id":2012167749,"title":"HANNA HI 38074 CTK - Boron Test Kit","handle":"hi38074","description":"\u003cdiv id=\"Product-Body\"\u003e\n\u003cp\u003eBoric acid\/borate react with chemical compounds containing multiple hydroxyls groups (polyols) such as mannitol. generating anionic complexes at the neutral pH of water.\u003c\/p\u003e\n\u003cp\u003eThe borate esters are formed and dissociated spontaneously in a variety of pH dependent equilibria. During to the release of acidic protons during complexation there is a concomitant decrease of pH which tends to reverse the reaction and thus. in order to maintain stable complexes there is a need to avoid pH decrease. The amount of acidification produced upon the addition of mannitol is proportional to the extent of borate ester formation.\u003c\/p\u003e\n\u003cp\u003eThe HI 38074 \u003cspan id=\"b7o32_2\" class=\"b7o32\"\u003etest\u003c\/span\u003e kit can determine boron concentration in irrigation waters by direct titration of boric acid.\u003c\/p\u003e\n\u003cp\u003e \u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003eHI 38074 test kit comes with reagent for 100 tests. HI 98103 Checker \u003cspan id=\"b7o32_3\" class=\"b7o32\"\u003epocket\u003c\/span\u003e pH meter. pH 4.01 (1 sachet). pH 7.01 (1 sachet). screwdriver. 120 mL bottle with cap. 50 mL calibrated vessel. and 1 mL plastic pipettes (2).\u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eSPECIFICATIONS:\u003c\/strong\u003e\u003cbr\u003e\u003c\/p\u003e\n\u003c\/div\u003e\n\u003cdiv id=\"TabbedPanels1\" class=\"TabbedPanels\"\u003e\n\u003cdiv class=\"TabbedPanelsContentGroup\"\u003e\n\u003cdiv class=\"TabbedPanelsContent TabbedPanelsContentVisible\"\u003e\n\u003ctable class=\"spec_table mceItemTable\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eTitration\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.0-5.0 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.2 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eBoric Acid\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e100\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e780 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e","published_at":"2024-05-14T12:25:56+12:00","created_at":"2015-09-04T15:16:06+12:00","vendor":"Hanna Instruments","type":"Electrochemical","tags":["Category_pH","Manufacturer_Hanna Instruments"],"price":18400,"price_min":18400,"price_max":18400,"available":true,"price_varies":false,"compare_at_price":null,"compare_at_price_min":0,"compare_at_price_max":0,"compare_at_price_varies":false,"variants":[{"id":5764241861,"title":"Default Title","option1":"Default Title","option2":null,"option3":null,"sku":"HI38074","requires_shipping":false,"taxable":true,"featured_image":null,"available":true,"name":"HANNA HI 38074 CTK - Boron Test Kit","public_title":null,"options":["Default Title"],"price":18400,"weight":0,"compare_at_price":null,"inventory_quantity":1,"inventory_management":null,"inventory_policy":"deny","barcode":null,"requires_selling_plan":false,"selling_plan_allocations":[]}],"images":["\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0483e22697_2015.jpeg?v=1465536676","\/\/acornsci.co.nz\/cdn\/shop\/products\/hi38074_200_2015.jpeg?v=1465536677"],"featured_image":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0483e22697_2015.jpeg?v=1465536676","options":["Title"],"media":[{"alt":"HI 38074 CTK - Boron Test Kit - Acorn Scientific","id":25183682659,"position":1,"preview_image":{"aspect_ratio":1.0,"height":1000,"width":1000,"src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0483e22697_2015.jpeg?v=1465536676"},"aspect_ratio":1.0,"height":1000,"media_type":"image","src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e0483e22697_2015.jpeg?v=1465536676","width":1000},{"alt":"HI 38074 CTK - Boron Test Kit - Acorn Scientific","id":25183715427,"position":2,"preview_image":{"aspect_ratio":1.0,"height":200,"width":200,"src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/hi38074_200_2015.jpeg?v=1465536677"},"aspect_ratio":1.0,"height":200,"media_type":"image","src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/hi38074_200_2015.jpeg?v=1465536677","width":200}],"requires_selling_plan":false,"selling_plan_groups":[],"content":"\u003cdiv id=\"Product-Body\"\u003e\n\u003cp\u003eBoric acid\/borate react with chemical compounds containing multiple hydroxyls groups (polyols) such as mannitol. generating anionic complexes at the neutral pH of water.\u003c\/p\u003e\n\u003cp\u003eThe borate esters are formed and dissociated spontaneously in a variety of pH dependent equilibria. During to the release of acidic protons during complexation there is a concomitant decrease of pH which tends to reverse the reaction and thus. in order to maintain stable complexes there is a need to avoid pH decrease. The amount of acidification produced upon the addition of mannitol is proportional to the extent of borate ester formation.\u003c\/p\u003e\n\u003cp\u003eThe HI 38074 \u003cspan id=\"b7o32_2\" class=\"b7o32\"\u003etest\u003c\/span\u003e kit can determine boron concentration in irrigation waters by direct titration of boric acid.\u003c\/p\u003e\n\u003cp\u003e \u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003eHI 38074 test kit comes with reagent for 100 tests. HI 98103 Checker \u003cspan id=\"b7o32_3\" class=\"b7o32\"\u003epocket\u003c\/span\u003e pH meter. pH 4.01 (1 sachet). pH 7.01 (1 sachet). screwdriver. 120 mL bottle with cap. 50 mL calibrated vessel. and 1 mL plastic pipettes (2).\u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eSPECIFICATIONS:\u003c\/strong\u003e\u003cbr\u003e\u003c\/p\u003e\n\u003c\/div\u003e\n\u003cdiv id=\"TabbedPanels1\" class=\"TabbedPanels\"\u003e\n\u003cdiv class=\"TabbedPanelsContentGroup\"\u003e\n\u003cdiv class=\"TabbedPanelsContent TabbedPanelsContentVisible\"\u003e\n\u003ctable class=\"spec_table mceItemTable\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eTitration\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.0-5.0 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.2 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eBoric Acid\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e100\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e780 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e\n\u003c\/div\u003e"}