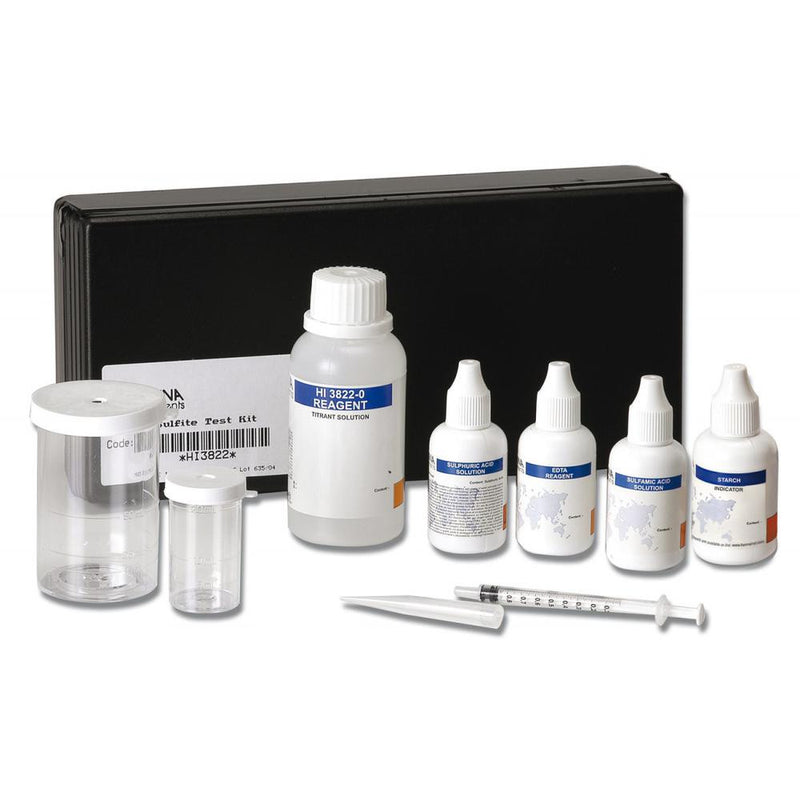
{"id":1582109253,"title":"HANNA HI 3822 - CTK Sulfite","handle":"hi-3822","description":"\u003cp\u003eThe HANNA sulfite test kit makes monitoring easy. quick and safe. The compact size gives the user the versatility to use the kit practically anywhere. The design of the kit makes it practically impossible to spill the reagents. thereby reducing the possibility of injury or damage to property.\u003c\/p\u003e\n\u003cp\u003eThe method used is an iodometric method. Iodide ions react with iodate ions in the presence of sulfuric acid to form iodine.\u003c\/p\u003e\n\u003cp\u003eThe sulfite present in the water sample then reduces the iodine back to iodide.\u003c\/p\u003e\n\u003cp\u003eAn excess of iodate ions will generate additional iodine. which will form a blue complex with starch. This color change determines the end point of this titration.\u003c\/p\u003e\n\u003cp\u003e \u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003e HI 3822 test kit comes with 30 mL sulfamic acid solution. 30 mL EDTA reagent. 15 mL sulfuric acid solution. 10 mL starch indicator. 120 mL titrant solution. 20 mL calibrated vessel. 50 mL calibrated vessel and calibrated syringe with tip.\u003c\/p\u003e\n\u003cdiv\u003e\u003cstrong\u003eProduct Manuals\u003c\/strong\u003e\u003c\/div\u003e\n\u003cp\u003e\u003cimg src=\"http:\/\/www.hannainst.com\/usa\/images\/icon-pdf.gif\" alt=\"\" width=\"20\" height=\"22\" align=\"absmiddle\" border=\"0\"\u003e Manual: \u003ca title=\"Download Product Manual\" href=\"http:\/\/www.hannainst.com\/manuals\/3822.pdf\" target=\"_blank\"\u003eDownload\u003c\/a\u003e\u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eSpecification :\u003c\/strong\u003e\u003c\/p\u003e\n\u003ctable class=\"spec_table\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eTitration\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.0-20.0 mg\/L\u003cbr\u003e 0-200 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.2 mg\/L\u003cbr\u003e 2 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eIodometric\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eapprox. 110\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e910 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cp\u003e \u003c\/p\u003e","published_at":"2016-09-01T16:06:24+12:00","created_at":"2015-09-01T15:08:50+12:00","vendor":"Hanna Instruments","type":"Electrochemical","tags":["Category_pH","Manufacturer_Hanna Instruments","Type_Buffers"],"price":18500,"price_min":18500,"price_max":18500,"available":true,"price_varies":false,"compare_at_price":null,"compare_at_price_min":0,"compare_at_price_max":0,"compare_at_price_varies":false,"variants":[{"id":4857184325,"title":"Default Title","option1":"Default Title","option2":null,"option3":null,"sku":"HI 3822","requires_shipping":false,"taxable":true,"featured_image":null,"available":true,"name":"HANNA HI 3822 - CTK Sulfite","public_title":null,"options":["Default Title"],"price":18500,"weight":0,"compare_at_price":null,"inventory_quantity":1,"inventory_management":null,"inventory_policy":"deny","barcode":null,"requires_selling_plan":false,"selling_plan_allocations":[]}],"images":["\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e05f9dabc85_2015.jpeg?v=1465536701"],"featured_image":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e05f9dabc85_2015.jpeg?v=1465536701","options":["Title"],"media":[{"alt":"HI 3822 - CTK Sulfite - Acorn Scientific","id":21280587875,"position":1,"preview_image":{"aspect_ratio":1.0,"height":1000,"width":1000,"src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e05f9dabc85_2015.jpeg?v=1465536701"},"aspect_ratio":1.0,"height":1000,"media_type":"image","src":"\/\/acornsci.co.nz\/cdn\/shop\/products\/31gluhj8kul__sl500_aa300_51e05f9dabc85_2015.jpeg?v=1465536701","width":1000}],"requires_selling_plan":false,"selling_plan_groups":[],"content":"\u003cp\u003eThe HANNA sulfite test kit makes monitoring easy. quick and safe. The compact size gives the user the versatility to use the kit practically anywhere. The design of the kit makes it practically impossible to spill the reagents. thereby reducing the possibility of injury or damage to property.\u003c\/p\u003e\n\u003cp\u003eThe method used is an iodometric method. Iodide ions react with iodate ions in the presence of sulfuric acid to form iodine.\u003c\/p\u003e\n\u003cp\u003eThe sulfite present in the water sample then reduces the iodine back to iodide.\u003c\/p\u003e\n\u003cp\u003eAn excess of iodate ions will generate additional iodine. which will form a blue complex with starch. This color change determines the end point of this titration.\u003c\/p\u003e\n\u003cp\u003e \u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eOrder Information:\u003c\/strong\u003e\u003cbr\u003e HI 3822 test kit comes with 30 mL sulfamic acid solution. 30 mL EDTA reagent. 15 mL sulfuric acid solution. 10 mL starch indicator. 120 mL titrant solution. 20 mL calibrated vessel. 50 mL calibrated vessel and calibrated syringe with tip.\u003c\/p\u003e\n\u003cdiv\u003e\u003cstrong\u003eProduct Manuals\u003c\/strong\u003e\u003c\/div\u003e\n\u003cp\u003e\u003cimg src=\"http:\/\/www.hannainst.com\/usa\/images\/icon-pdf.gif\" alt=\"\" width=\"20\" height=\"22\" align=\"absmiddle\" border=\"0\"\u003e Manual: \u003ca title=\"Download Product Manual\" href=\"http:\/\/www.hannainst.com\/manuals\/3822.pdf\" target=\"_blank\"\u003eDownload\u003c\/a\u003e\u003c\/p\u003e\n\u003cp\u003e\u003cstrong\u003eSpecification :\u003c\/strong\u003e\u003c\/p\u003e\n\u003ctable class=\"spec_table\"\u003e\n\u003ctbody\u003e\n\u003ctr\u003e\n\u003ctd\u003eMethod\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eTitration\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eRange\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.0-20.0 mg\/L\u003cbr\u003e 0-200 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eSmallest Increment\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e0.2 mg\/L\u003cbr\u003e 2 mg\/L\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eChemical Method\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eIodometric\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eNumber of Tests\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003eapprox. 110\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003ctr\u003e\n\u003ctd\u003eWeight\u003c\/td\u003e\n\u003ctd\u003e \u003c\/td\u003e\n\u003ctd\u003e910 g\u003c\/td\u003e\n\u003c\/tr\u003e\n\u003c\/tbody\u003e\n\u003c\/table\u003e\n\u003cp\u003e \u003c\/p\u003e"}
HANNA HI 3822 - CTK Sulfite
Related Products
HIRSCHMANN 9490124 PIPETTE TIPS 1-5ML
$155.00 $190.00